Battery Fundamentals: What Is Battery
Batteries are electrochemical devices that store chemical energy and convert it into electrical energy. They are essential components of many modern technologies, powering everything from smartphones and laptops to electric vehicles and grid-scale energy storage systems.
Battery Components
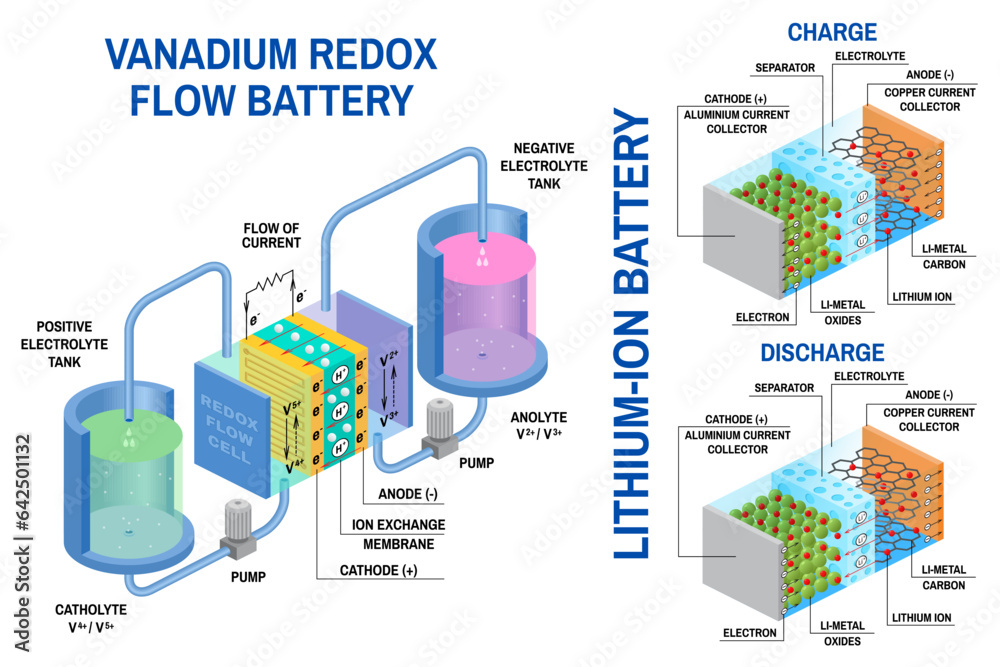
The fundamental components of a battery are the electrodes, electrolyte, and separator. These components work together to facilitate the chemical reactions that produce electrical energy.
- Electrodes: These are conductive materials that serve as the sites where chemical reactions occur. There are two types of electrodes: the anode and the cathode. The anode is the negative electrode, where oxidation occurs, releasing electrons. The cathode is the positive electrode, where reduction occurs, accepting electrons.
- Electrolyte: This is a solution or paste that allows the flow of ions between the electrodes. The electrolyte is crucial for completing the electrical circuit within the battery.
- Separator: This is a porous membrane that physically separates the anode and cathode, preventing direct contact between them. The separator allows the flow of ions while preventing short circuits.
Chemical Reactions in Battery Operation
The operation of a battery is based on a series of chemical reactions that involve the transfer of electrons between the electrodes and the electrolyte. These reactions are reversible, allowing the battery to be charged and discharged.
The chemical reactions involved in battery operation can be represented by the following general equations:
Discharge: Anode (oxidation) + Cathode (reduction) → Products + Electrical Energy
Charge: Products + Electrical Energy → Anode (reduction) + Cathode (oxidation)
- Discharge: During discharge, the anode undergoes oxidation, releasing electrons that flow through the external circuit to the cathode. The cathode undergoes reduction, accepting these electrons and reacting with ions from the electrolyte. This process generates an electrical current that powers the connected device.
- Charge: During charging, the process is reversed. An external source of electrical energy forces electrons to flow from the cathode to the anode. This causes the anode to undergo reduction and the cathode to undergo oxidation, restoring the battery to its original state.
Types of Batteries
There are numerous types of batteries, each with its own unique chemistry, performance characteristics, and applications. Some common types include:
- Lead-acid batteries: These are the oldest and most widely used type of battery. They are commonly found in automobiles, motorcycles, and backup power systems.
- Lithium-ion batteries: These are high-energy-density batteries that are commonly used in portable electronic devices, electric vehicles, and grid-scale energy storage.
- Nickel-cadmium (NiCd) batteries: These are rechargeable batteries that were once widely used in cordless tools and other applications. However, their use has declined due to environmental concerns.
- Nickel-metal hydride (NiMH) batteries: These are a more environmentally friendly alternative to NiCd batteries and are often used in hybrid vehicles and portable electronic devices.
Types of Batteries

Batteries are essential components of many modern devices, powering everything from smartphones and laptops to electric vehicles and grid-scale energy storage. They convert chemical energy into electrical energy, providing a portable and reliable source of power.
Battery Types
The diverse world of batteries encompasses a range of chemistries, each with its own unique characteristics and applications. Understanding these distinctions is crucial for selecting the appropriate battery for a specific need.
- Lead-Acid Batteries: These are the oldest and most widely used battery type. They consist of lead plates immersed in an electrolyte solution of sulfuric acid. Lead-acid batteries are known for their low cost, high current output, and ability to withstand deep discharges. However, they are also heavy, bulky, and have a relatively short lifespan.
Lead-acid batteries are commonly used in automotive applications, such as starting, lighting, and ignition (SLI) systems, as well as in backup power systems for computers and other sensitive equipment.
- Lithium-Ion Batteries: Lithium-ion (Li-ion) batteries are among the most popular battery types due to their high energy density, long lifespan, and lightweight design. They utilize lithium ions that move between the anode and cathode, generating electrical current.
Li-ion batteries power a wide range of devices, including smartphones, laptops, electric vehicles, and energy storage systems. Their high energy density allows for compact and lightweight designs, making them ideal for portable electronics.
- Alkaline Batteries: Alkaline batteries are primary batteries, meaning they are not rechargeable. They employ a zinc anode and a manganese dioxide cathode in an alkaline electrolyte. Alkaline batteries are known for their long shelf life, high energy density, and affordability.
Alkaline batteries are commonly used in everyday devices such as toys, remote controls, and flashlights. Their long shelf life makes them suitable for applications where frequent use is not required.
Comparison of Battery Characteristics
The following table provides a comparison of the key characteristics of different battery types:
| Characteristic | Lead-Acid | Lithium-Ion | Alkaline |
|---|---|---|---|
| Energy Density | Low | High | Moderate |
| Lifespan | Short | Long | Short |
| Cost | Low | Moderate | Low |
| Safety | Moderate | Moderate | High |
| Environmental Impact | Moderate | Moderate | Moderate |
Applications of Battery Types, What is battery
The choice of battery type depends on the specific application and its requirements. The following table highlights the advantages and disadvantages of different battery types in various applications:
| Application | Battery Type | Advantages | Disadvantages |
|---|---|---|---|
| Automotive (SLI) | Lead-Acid | Low cost, high current output | Heavy, bulky, short lifespan |
| Portable Electronics (Smartphones, Laptops) | Lithium-Ion | High energy density, long lifespan, lightweight | Moderate cost, potential safety concerns |
| Electric Vehicles | Lithium-Ion | High energy density, long lifespan | High cost, potential safety concerns |
| Grid-Scale Energy Storage | Lithium-Ion | High energy density, long lifespan | High cost, potential safety concerns |
| Everyday Devices (Toys, Remote Controls) | Alkaline | Low cost, long shelf life | Not rechargeable, moderate energy density |
Battery Applications
Batteries have become an indispensable part of modern life, powering a wide array of devices and systems. Their ability to store and release energy makes them essential for various applications, from portable electronics to large-scale energy storage.
Electronics
Batteries are the lifeblood of our electronic devices, enabling portability and convenience. From smartphones and laptops to wearables and medical devices, batteries provide the power needed for these devices to function. Lithium-ion batteries, known for their high energy density and long lifespan, are widely used in these applications.
Transportation
The rise of electric vehicles (EVs) has revolutionized the automotive industry, with batteries playing a pivotal role. EVs rely on large battery packs to store energy and power the electric motors. These batteries offer several advantages over traditional gasoline engines, including reduced emissions and improved fuel efficiency.
Energy Storage
Batteries are crucial for energy storage, enabling the transition to a more sustainable energy future. They can store excess energy generated from renewable sources, such as solar and wind power, and release it when needed, ensuring a consistent supply of electricity. Battery energy storage systems (BESS) are increasingly being deployed to enhance grid stability and reliability.
Innovative Battery Applications
Beyond these traditional applications, batteries are driving innovation in various fields.
Electric Vehicles
Electric vehicles (EVs) are a prime example of innovative battery applications. The development of high-capacity, long-lasting batteries has been crucial for the success of EVs. The range and performance of EVs have improved significantly, making them a viable alternative to gasoline-powered vehicles.
Renewable Energy Systems
Batteries play a critical role in renewable energy systems, enabling the storage and utilization of energy generated from sources like solar and wind power. This allows for a more reliable and consistent supply of electricity, even when these sources are intermittent.
Medical Devices
Batteries power a wide range of medical devices, including pacemakers, insulin pumps, and hearing aids. These devices rely on batteries for their functionality and provide essential support to patients. The development of smaller, more efficient batteries has improved the performance and longevity of these devices.
Potential Future Applications
The future of battery technology holds immense potential, with applications extending beyond current uses.
Grid-Scale Energy Storage
Batteries will play an increasingly vital role in grid-scale energy storage, enabling the integration of renewable energy sources and enhancing grid stability. Large-scale battery storage systems can provide a buffer against fluctuations in energy supply and demand, ensuring a reliable and consistent flow of electricity.
Off-Grid Power Systems
Batteries are essential for off-grid power systems, providing a reliable source of electricity in remote areas or during power outages. They can be used to power homes, businesses, and communities that are not connected to the traditional electricity grid.
Smart Homes
Batteries will be integral to smart homes, powering various devices and appliances, and enabling energy efficiency. They can store energy generated from solar panels or other renewable sources, reducing reliance on the grid and lowering energy costs.
What is battery – A battery is a device that stores chemical energy and converts it into electrical energy. It’s a fundamental component in many devices, from smartphones to electric cars. While we’re on the topic of energy, did you hear the news about Skai Jackson being pregnant, as confirmed by TMZ ?
It’s exciting news for her, and it reminds us that life, like a battery, has its own charge and potential for growth.
A battery is a device that stores chemical energy and converts it into electrical energy. This process is similar to how relationships, like the one between skai jackson and boyfriend , can be energized by the exchange of emotions and experiences.
Just as a battery eventually runs out of power, even the strongest relationships require nurturing and attention to maintain their spark.
